Recommended
CGD NOTES

Blog Post

Blog Post
This note is part of a body of research that examines the current state of play on COVID-19 treatments. These pieces provide a deep dive into key cross-cutting areas—demand, voluntary licensing for generic supply, and deployment—and offer policy actions for 2023 and beyond. See here for more from CGD on COVID-19 treatments.
Introduction
Ensuring rapid and equitable access to effective oral COVID-19 therapies is essential to reducing health disparities from COVID-19 and building a better response system for future epidemics and pandemics. A multipronged strategy for medical countermeasures must include antivirals, monoclonal antibodies, and other mechanisms to fight infections. Better strategies to deploy oral antivirals, which have been important in the context of COVID-19, will also help to prepare the system to deploy medical countermeasures at a large scale in future outbreaks.
As with many new health technologies, the initial global supply of the two main oral antivirals currently authorized for COVID-19—Nirmatrelvir/ritonavir (Pfizer’s Paxlovid) and Molnupiravir (Merck’s Lagevrio)—was limited, and efforts focused on increasing overall supply. Pfizer announced an agreement in September 2022 to donate 6 billion courses of Paxlovid to the Global Fund, to be made available for procurement by 132 eligible countries. Although it is essential to have a diversified manufacturing base for antivirals and some ready-to-go capacity, important questions are arising around a strategy to rapidly deploy oral antivirals on a global scale, including in regions with lower vaccination rates. In the past month, two test-to-treat pilot programs in low- and middle- income countries (LMICs) have been announced: one by the COVID Treatment Quick Start Consortium and the second by USAID. Meanwhile, deployment strategies in the United States and other high-income countries are experiencing challenges.
In this note, we explore lessons learned from the US experience deploying COVID-19 oral antivirals and the unique considerations for LMIC delivery and access. We present three key actions that can aid in the development of an oral antiviral deployment strategy in low-resource settings globally.
The US experience with COVID-19 oral antivirals deployment
The US government launched the Test to Treat Initiative in early March 2022 to increase access to lifesaving COVID-19 treatment, including oral antivirals. The initiative is designed to provide access through three simple steps: (1) get tested (at-home rapid tests or results from a “One-Stop Test to Treat” location is sufficient), (2) receive a prescription on site, and (3) fill the prescription and begin treatment. Test-to-Treat locations include pharmacy-based clinics, federally funded health centers, long-term care facilities, and federally supported test-to-treat sites, which began to further expand access in May 2022.
All sites dispensing oral antivirals report their dispensing data (inventory and utilization data) daily into the HHS Health Partner Ordering Portal. The allocation and distribution of COVID-19 therapeutics is managed by federal agencies by utilizing the Tiberius platform, which brings together data from different ordering sites, providers, and suppliers to create a dashboard view of demand and supply.
Deployment challenges in the US
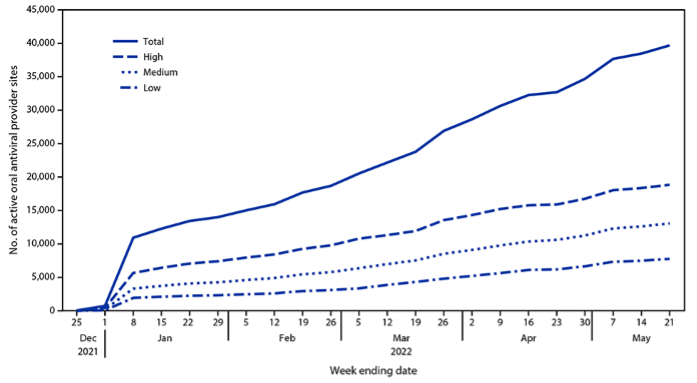
In its starting phase, the test-to-treat program faced numerous challenges. Because COVID-19 oral antivirals must be taken within five days of the onset of symptoms to be effective, an access strategy based on community convenience and ease of access is of the utmost importance. While primary care providers (PCPs) are the main access point for patients to seek treatment and receive a prescription, several impediments may deter patients from obtaining their medication. Accordingly, the US Test to Treat Initiative was designed as a one-stop shop for testing, obtaining a prescription, and receiving treatment. In May 2022, 87 percent of the dispensing sites in the Test-to-Treat Initiative were retail pharmacies, which made filling a prescription easier. Concerted efforts were made to include more dispensing sites and test-treat locations in communities with higher social vulnerability, as shown in Figure 1.
Figure. 1 Number of active provider sites for oral antiviral therapy against COVID-19, by week and zip code social vulnerability score (United States, December 23, 2021–May 21, 2022)
Note: Zip codes classified as having low, medium, or high social vulnerability based on ranking within the lower, middle, and upper tertiles of an Equitable Distribution Index score.
Source: Gold JA, Kelleher J, Magid J, et al., Dispensing of Oral Antiviral Drugs for Treatment of COVID-19 by Zip Code–Level Social Vulnerability — United States, December 23, 2021–May 21, 2022. CDC, Morbidity and Mortality Weekly Report June 24, 2022.
Many patients at higher risk of severe illness from COVID-19 have comorbidities treated with medications that may interfere with the way COVID-19 oral antivirals should act in the body—a phenomenon known as drug-drug interactions (DDIs). Paxlovid has multiple DDIs, meaning it cannot be prescribed for patients with certain conditions unless the dosing of their other medicines is altered. Because of the potential of DDIs with the COVID-19 oral antivirals, designing a safe deployment mechanism requires more information than a positive test and a subsequent disbursement of medication. A qualified health professional, typically a PCP, must consider potential DDIs and determine whether an individual can safely take the COVID-19 oral antiviral in combination with their other medications. But most pharmacies serving as dispensing sites do not have authorized PCP prescribers available on-site or via telemedicine.
Whether point-of-care prescription of COVID-19 oral antivirals should also be delegated to pharmacists, particularly for Paxlovid, is subject to debate. Pharmacist groups such as the American Pharmacists Association (APhA) have urged the FDA to allow them to prescribe COVID-19 antivirals, noting that in June 2022, there were about 28,000 community pharmacies in federally recognized underserved communities but just 838 test-to-treat sites; utilizing these pharmacies could increase access to treatments by up to 3,200 percent, according to the APhA. While there was concern about pharmacists managing DDIs, on July 6, 2022, the FDA authorized pharmacists to prescribe Paxlovid under certain conditions: pharmacists must review patients’ health records for kidney or liver problems and a list of all medications they are taking to screen for DDI with Paxlovid. Pharmacists are still unable to prescribe Molnupiravir at the time of this writing.
Another challenge in the US is the payment model for COVID-19 oral antivirals—pharmacies are making very little profit. Because the medications are paid for by the federal government, essentially making them “free,” pharmacies are only paid a dispensing fee for the drugs. Although the Centers for Medicare and Medicaid Services instructed pharmacy benefit managers (PBMs) to pay sufficient dispensing fees for the oral antivirals, especially under the unique circumstances of the pandemic, in February 2022, pharmacies were being paid anywhere from $1 to $10.50 by PBMs. These fees are significantly lower than what pharmacies are paid for COVID-19 vaccines, for example, which on average is about $40. Such circumstances could reduce a pharmacy’s incentive to stock and dispense oral antivirals.
How will these challenges manifest in resource-limited health systems around the world?
The challenges observed in the US may be reflected in LMCs but with nuances that are unique to each health system. The test-to-treat architecture developed by ACT-A and other global agencies centers on public sector primary health centers (PHCs) and clinics. Such health facilities have overcrowded outpatient services, which poses three problems. First, with overcrowding in the public sector PHCs, it may be more difficult for patients to receive and initiate treatment within five days of their symptom onset, as recommended for both Paxlovid and Molnupiravir. Second, overcrowding in hospitals and health centers can increase the risk of infection, especially given the highly transmissible nature of COVID-19. Extra precautions would have to be taken to ensure that social distancing, masks, and other preventative measures are utilized to reduce the spread of the virus. Third, even if infection risks and patient wait times can be managed, congesting PHCs with suspected COVID-19 cases may lead to delayed care initiation for other acute conditions for which patients are visiting the PHC.
Ease of access to COVID-19 oral antivirals should be a central component to a deployment strategy. As much as 80 percent of health products in lower-middle income countries like Nigeria, Ghana, India, and Kenya are purchased from private pharmacies. Furthermore, in these contexts, most individuals go to private pharmacies and drug shops to seek treatments for febrile illnesses. Authorizing private pharmacies to prescribe and dispense COVID-19 antivirals would likely improve access to these medications, as it aligns with patient behaviors and preferences. At the same time, the lack of regulation and staff competency to provide point-of-care testing and diagnostic services, raises concerns about the quality of care, particularly with respect to DDIs. While these concerns are legitimate, measures are being taken to improve distribution, price, quality, and availability at these locations. Additionally, innovative solutions to counter these issues are already being developed. Entrepreneurship within the pharmaceutical sector is on the rise in African markets, and the advantages that these organizations have acquired, especially in regard to technology and digital marketplaces, make them essential actors within a future deployment strategy.
Antiretroviral therapy (ART) programming in multiple countries has revealed the convenience and congestion problem that arises when dispensing of ARVs is limited to PHCs. As a result, differentiated service delivery (DSD) models have been developed that bring dispensing points closer to the patients through designated pick-up points or through community health workers.
Another potential challenge in LMICs is the lack of a centralized reporting system. Governments and donors are working to build systems where private clinics and pharmacies report utilization and other relevant data into national health information management systems such DHIS2 or medicine use databases. But in most countries, such attempts have achieved limited success and there is no systematic reporting of data by private pharmacies into the national system. This makes it harder to bring test to treat programs to private pharmacies as in the US and other OECD countries.
What can be done to address these challenges?
To address these challenges in resource-limited settings, we propose three key actions to aid in the development of an oral antiviral deployment strategy:
1. Leverage private pharmacies and digital tools for MCM deployment
Addressing the unique challenges for an oral antiviral deployment strategy in resource-limited settings requires an approach that leverages both public and private channels to ensure the widest reach and fast response times. Building test to treat initiatives on top of current telemedicine and digital health systems can help to reduce overcrowding at PHCs and limit the spread of infection. Under such digital test to treat initiatives, individuals first test for COVID-19 and have a telehealth visit with a provider (doctor or pharmacists). Next, depending on dispensing regulations in a country, they receive a prescription for an oral antiviral treatment (if they are eligible), and have the prescription filled—all in one location. Telemedicine, combined with retail pharmacy dispensing and/or direct-to-consumer distribution, is one the most common types of services offered by startups and innovators operating in the health sector in Africa; mPharma, MYDAWA, SASAdoctor, and RocketHealth Uganda are some of the lead operators in this space.
Adding a simple patient-facing interface allows patients to initiate the process for receiving COVID-19 treatment by entering the symptoms, the date their symptoms began, and any other relevant medical information, such as the medications they are currently taking (both over the counter and prescribed). Such a system can utilize DDI tracker tools and an embedded treatment algorithm to recommend the right course of treatment and to support decision making in prescribing oral COVID-19 antivirals.
Telemedicine (diagnosis with provider consultation) by itself can lessen the burden on health systems by keeping patients at home and not overcrowding hospitals. However, patients would still need to travel to a pharmacy to fill their prescriptions and begin treatment.
The inclusion of private pharmacies, chemists, and drug shops in a deployment strategy would assist in alleviating the difficulty of finding a pharmacy to fill such prescriptions. mPharma, Maisha Meds, SwipeRx, MYDAWA, and Kasha are examples of digital-first retail pharmacy networks or pharmacy service providers that use technology to deliver medications from high-quality manufacturers to retail pharmacies.
Admittedly, many of these operations are limited to urban and peri-urban areas and will not allow reach into rural areas and poor populations. But the goal in such an initiative is not necessarily national reach right at the start, but instead to start a program that allows priming private pharmacies and digital technology as an additional channel for access to medical countermeasures.
2. Enhance tracking of oral antiviral distribution and dispensing, especially in the private channel
Tracking distribution and dispensing of oral antivirals in the private channel requires collaboration between public and private health reporting systems and integration of existing and newly developed IT systems. Attempts in the past to make private pharmacies (and clinics) report into national systems have failed because there were weak incentives for pharmacies to adopt such practices. However, in a test-to-treat program in private pharmacies, the pharmacies have a stronger incentive to provide information about on-hand inventory, dispensing, and utilization into a public system because the medicines and diagnostics are provided either free or heavily subsidized by the government (and its health sector development partners).
The benefits of making such a reporting system work for an oral antiviral test-to-treat program are significant in terms of establishing a channel of reporting that currently does not exist, leaving the private channel to operate in a manner that is disconnected from the main health system. Technology systems already exist and capture such dispensing data in private pharmacies that are part of the digital networks operated by mPharma, Maisha Meds, SwipeRx, MyDawa and others. There are also simple APIs that can help add the data collected from the private pharmacy systems into national public data on distribution, dispensing, and utilization. Once again, even a specific integration of private pharmacy dispensing data into national health systems (i.e. for oral antivirals for COVID-19) would jump start the process of more systematic integration, which is crucial not only for its use as a rapid dispending channel for medical countermeasures, but more broadly for achieving universal health coverage in mixed health systems.
3. Utilize and strengthen existing global health infrastructure to support test-to-treat initiatives in the private channel
While national governments play a specific and overarching role in the distribution of health products more broadly, utilizing partners with existing infrastructure could lessen the burden on national governments and expand access more rapidly. To the extent possible, this should be carried out by leveraging national government systems and staff. In addition, existing resources for global health programs can also be utilized. For Global Fund eligible countries, the purchase of oral antivirals (and diagnostics) could use Global Fund resourcing and Global Fund procurement systems. These products could then be made available to pharmacy networks such as the ones mentioned earlier as part of a partnership agreement under which the pharmacy networks will provide visibility of dispensing, commit to retail prices for test and treat that are based on a fixed dispensing/test-treat program fee instead of charging a retail price to the patient. They will also commit to reporting data into national systems.
In addition to providing resourcing for purchase of antivirals and diagnostics, global health programming could also be utilized for providing operational assistance to run such a program. Existing global health programs have attempted private channel engagement in different shapes and forms. For example, the in-country staff for PEPFAR (the President’s Emergency Plan for AIDS Relief), PMI (the President’s Malaria Initiative), and USAID/TB along with the Global Fund and Stop TB Partnership could apply their experience in supporting test-to-treat initiatives for malaria, DSD for antiretrovirals, and public-private mix (PPM) for TB to a new initiative for COVID-19 oral antivirals and more. The two recommendations described above can be embedded in the programs run by global agencies for DSD and PPM, and as a result would rely on existing project infrastructure to get this oral antiviral initiative jumpstarted. To be effective, this would need to be coupled with strong research and evidence components. As these programs begin in a few countries, a learning network can be established across countries and implementation partners. The learning network can be used to share insights on dispensing practices, incentive structures, willingness of pharmacy networks to join test and treat programs, socio-economic profiles of patients reached, and other best practices.
Conclusion
Incorporating oral antiviral medications into an infectious disease outbreak response strategy can combat illness, hospitalizations, and death. After supply concerns are addressed, a focus on innovative deployment strategies is needed. While the US faced several challenges with its deployment of Paxlovid and Molnupiravir, the Test to Treat Initiative provides important lessons about oral antiviral deployment across country contexts, particularly in low-resource settings.
While some challenges in the US context may be also be experienced in low-resource settings, these countries may also face unique challenges such as overcrowding, and the essential role of private pharmacies for rapid access require tailored solutions. By utilizing digital tools, delivering medical countermeasures through private locally owned pharmacies, and creating a stronger bridge between private and public reporting systems, some of these hurdles can be addressed. Investing in deployment infrastructure will benefit patients and providers during this pandemic and will help to create a deployment architecture for oral antivirals and other medical countermeasures needed in the future. Some of these activities do not require investing from scratch—rather, where possible they can be built on top of existing investments in PPM for TB and DSD for ARVs, and product purchase agreements. This may not necessarily achieve significant impact for COVID-19 health outcomes, but it will help jumpstart a program and help refine the design for a test to treat program for the private pharmacy channel, which is often the dominant first point of care for patients in many LMICs.
Rights & Permissions
You may use and disseminate CGD’s publications under these conditions.