Recommended
Every year, almost five million people die with an infection that is resistant to the drugs used to treat it. A quarter of these people die because the drugs used to treat them do not work. Whilst the burden of resistance is high across the world, it falls greatest on the world’s poorest. A child born in Africa is 58 times more likely to die of a resistant infection before their fifth birthday than a child born in a high-income country. About half of these deaths (nearly 140,000) occur in the first month of life. The world has failed these children and the many others who die needlessly.
The current system for antimicrobial procurement, where manufacturers are paid per drug sold, is not fit for purpose. It provides insufficient and inequitable access to antimicrobials, incentivises overuse and inappropriate use, and fails to adequately fund R&D for new drugs to replace those rendered ineffective by the spread of resistance. Overcoming AMR requires re-envisioning the way antimicrobials are procured, including in low- and middle-income countries (LMICs). We need better purchasing systems that generate the incentives and investments to fund antimicrobial innovation, reduce unnecessary antibiotic use and increase stewardship, and, above all, secure access for those who need them. Far too many people die for want of a drug that already exists.
To this end, CGD has set up a working group, A New Grand Bargain for Antimicrobial Procurement: Improving Purchasing Systems to Enhance Access, Stewardship, and Innovation for Antimicrobials in Low- and Middle-Income Countries (LMICs). The group will examine different ways to purchase antimicrobials in LMICs in order to identify actionable policies to improve access and stewardship for key products and increase funding for research into new ones. Ultimately, governments, civil society, industry, health providers, and patients need to strike a new grand bargain to govern the rights and responsibilities to develop, protect, and ensure access to these vital medicines.
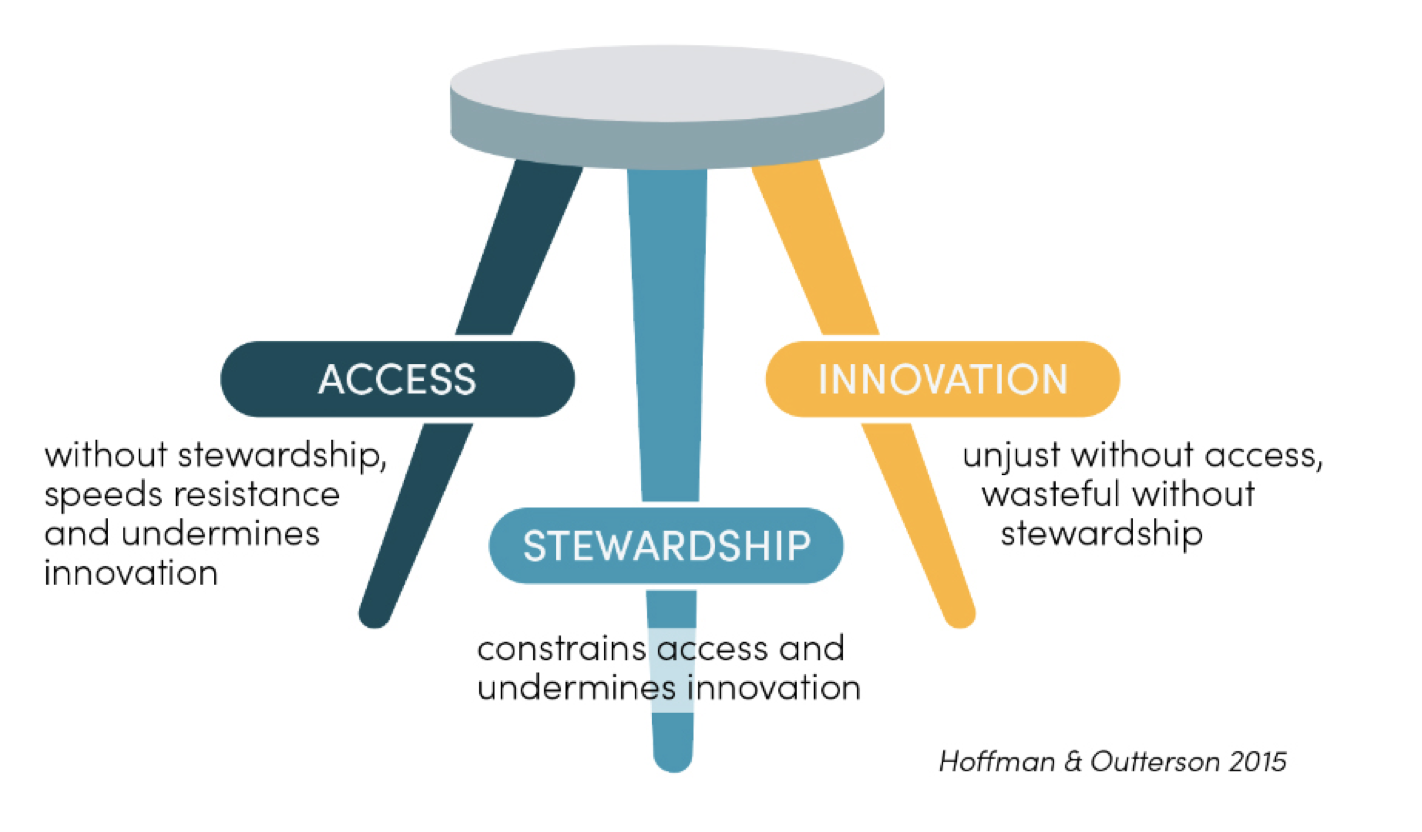
To lay the foundations of this project, we are launching today the Working Group’s first working paper, Leveraging Purchasing Systems to Ensure Access, Stewardship, and Innovation: A Landscape Review of Current and Potential Market Structures for Antimicrobials. This paper is the outcome of a systematic review of academic and grey literature as well as expert interviews. It synthesises existing research and thinking on how antimicrobials are procured and ways to improve the current purchasing system. It also examines interventions designed to improve innovation, access, and stewardship of antimicrobials and achieve a careful balance between these three interdependent components (see Figure 1).
Figure 1. A successful procurement system needs to provide access, stewardship, and innovation
Our paper includes three key findings:
-
The literature on antimicrobial procurement is overly focused on high-income countries (HICs). Whilst 51 percent of papers mention an LMIC, fewer than 10 percent exclusively focus on LMICs. Also, less that 13 percent of papers with listed authors include an author based in an LMIC. Priorities in LMICs and HICs differ, as evidenced in both the interviews we conducted and the literature. LMIC groups focus more on access to drugs, while HIC groups are more concerned about innovation. Both groups highlighted stewardship as a priority. To quote one interviewee, “There’s a divergence of interests between the Global North and South. The Global North is interested in new antibiotic production, R&D, stewardship, and surveillance. This is being done to identify threats to the Global North—not to help the Global South. The Global South is more interested in infection burden and reducing infectious disease.”
-
There is broad agreement that a new purchasing system is needed for antimicrobials in LMICs. Although there’s no consensus in the literature about the best way to reform purchasing systems, interview findings suggest a more recent coalescence around subscription models in HICs. In these models, purchasers pay annually for a drug, regardless of how many units are needed. The National Health System in the United Kingdom is currently piloting such a system with two drugs, and the US Congress is considering its own version with the PASTEUR Act. There is less clarity on the optimal system for LMICs.
-
There is insufficient research on how to implement policies and—with the exception of the GAIN Act, a 2012 piece of US legislation that grants an additional five years of exclusivity for qualifying antimicrobials—a dearth of research evaluating previously implemented initiatives.
What’s next
In the coming months, the working group will publish three country case studies that will examine in-depth the purchasing systems and policy landscape for new procurement arrangements in each setting. Case studies are confirmed for Brazil and India, and we are scoping a third partnership in sub-Saharan Africa.
The working group will also release a range of smaller research products—led by CGD’s research team or working group members—that will explore relevant research questions to inform working group deliberations and broader discussions.
A draft final report for public consultation will be published in April 2023, and the working group process will culminate in a final report, launched at the time of the UN General Assembly in September 2023, that will provide actionable policy recommendations about the design and implementation of purchasing systems for LMICs.
Working group members:
- Javier Guzman, Center for Global Development, Chair
- Anthony McDonnell, Center for Global Development, Technical Lead
- Manica Balasegaram, GARDP
- Peter Beyer, World Health Organisation
- Siddhartha Bhattacharya, NATHEALTH
- Thomas Cueni, International Federation of Pharmaceutical Manufacturers and Associations
- Austen Davis, Norwegian Agency for Development Cooperation
- Amanda Glassman, Center for Global Development
- Steve Isaacs, Aduro BioTech
- Jayasree Iyer, Access to Medicine Foundation
- Mahlet Kifle Habtemariam, Africa Centres for Disease Control and Prevention
- Jeremy Knox, Wellcome Trust
- Mirfin Mpundu, ReAct Africa
- Badri Narayanan, National Institution for Transforming India
- Tochi Okwor, Nigeria Centre for Disease Control
- Kevin Outterson, CARB-X
- Milton Ozorio Moraes, Fiocruz
- John Rex, F2G
- Naomi Rupasinghe, World Bank
- Rachel Silverman, Center for Global Development
- Faisal Sultan, Former Special Assistant to the Prime Minister of Pakistan
- Yot Teerawattananon, HITAP
- Brenda Waning, Stop TB Partnership at UNOPS
- Prashant Yadav, Center for Global Development
CGD research staff
- Katherine Klemperer
- Morgan Pincombe
Disclaimer
CGD blog posts reflect the views of the authors, drawing on prior research and experience in their areas of expertise. CGD is a nonpartisan, independent organization and does not take institutional positions.